Ionic Equation of Magnesium and Hydrochloric Acid
First we balance the molecular equation. Magnesium reacts with hydrochloric acid according to the equation.

Write The Net Ionic Equation In Which The Slightly Soluble Salt Magnesium Oxalate Mgc2o4 Dissolves In Dilute Hydrochloric Acid The Boxed Equation Below Is The Correct Answer But I Don T Get How
But the definition of.
. For example the ionic equation. 2H aq Mgs Mg 2 aq H 2 g This ionic equation can be split into two half equations. There are three main steps for writing the net ionic equation for Mg HCl MgCl2 H2 Magnesium Hydrochloric acid.
Answer 1 of 3. Mgs 2HClaq MgCl 2 aq H 2 g Explanation. 2 Br-aq Pb2aq PbBr 2 s 15.
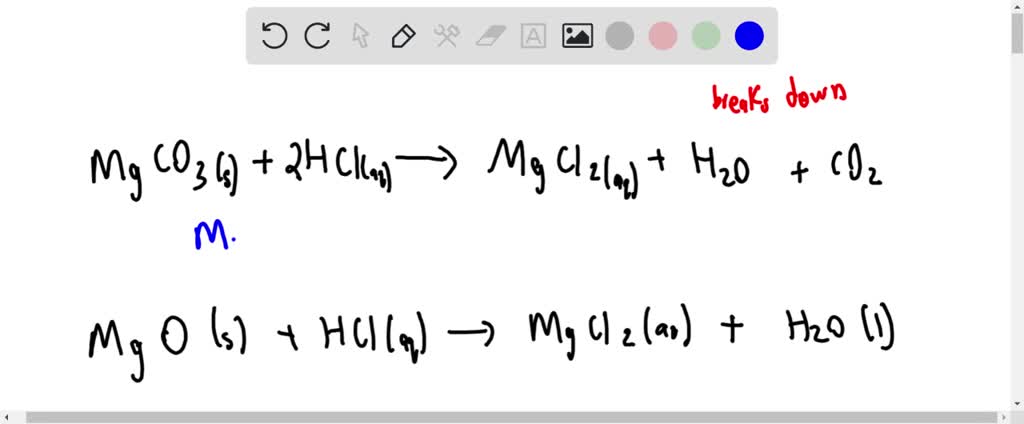
2HClaq MgCO 3 s MgCl 2 aq H 2 Ol CO 2 g. Mg2H2Cl---Mg22Cl-H2 Cancel out the Clsbecause they dont change and your ionic equation isMg2H. Magnesium reacts with hydrochloric acid according to the equation.
For example when hydrochloric acid HCl is mixed with potassium hydroxide. The overall reaction is MgO 2HCl - MgCl2 H2O. Magnesium reacts with hydrochloric acid according to the equation.
Ionic equation of magnesium added to dilute hydrochloric acid. Second we write the states and. A net ionic equation shows all the ionic substances as ions and shows the correct state of Answer.
2 F-aq Mg2aq MgF 2 s 16. For the reaction of magnesium with hydrochloric acid is. Magnesium metal hydrochloric acid rarr magnesium chloride dihydrogen gas Magnesium metal is oxidized to Mg2.
Mg s 2 HCl aq â MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single substitution reaction or to demonstrate the generation of hydrogen gas. For each of the following write i a balanced chemical complete ionic equation and it a net ionic equation. To balance the equations and ionic equation write a balanced chemistry equation and an equation.
The acids hydrogen ions react with the alkalis hydroxide ions to produce water. Mgs 2HClaq rarr MgCl_2aq H_2guarr Are all. This reaction is an example of a single replacement.
2 Haq 2 Br-aq Pb2aq 2ClO 4-aq 2Haq 2 ClO 4-aq PbBr 2 s NIE. Mgs 2 HClaq --MgCl2aq H2g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to. Magnesium chloride and hydrogen gas are created as a result of the reaction between magnesium and hydrochloric acid.
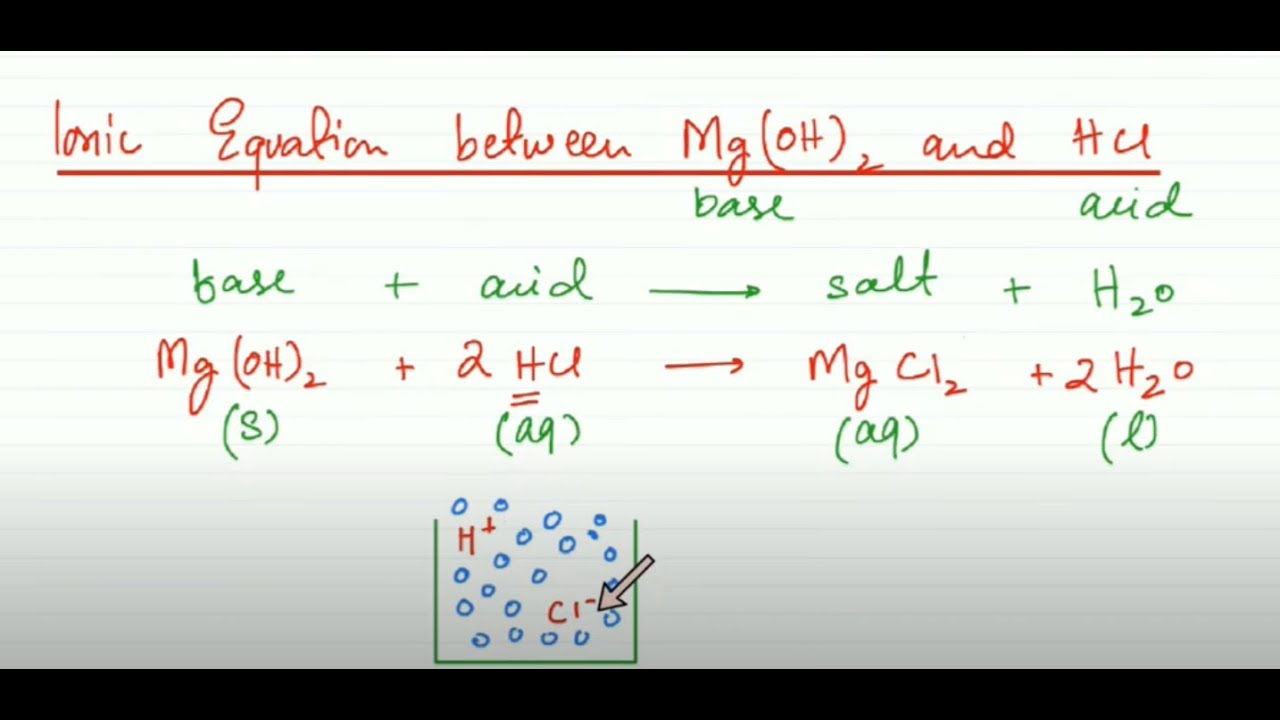
AnswerMgs 2Haq Mg²aq H₂g Explanation. All metal oxides are basic and as such an acid-base neutralisation reaction takes place with Base Acid - Salt Water. Write a balanced chemical equation for this reaction.
Mgs rarr Mg2 2e- Protium ion is REDUCED to dihydrogen gas. Mgs 2 HClaq -- MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gas. 2 KFaq MgNO 3 2 aq 2 KNO 3 aq MgF 2 s Ionic Equation.
Aqueous sulfurous acid H2SO3 and aqueous sodium chloride are formed by the reaction of aqueous sodium sulfite Na2SO3 and aqueous hydrochloric acid HCl. Write the ionic equation for the acid-carbonate reaction between hydrochloric acid and insoluble magnesium carbonate to form magnesium chloride salt water and carbon dioxide. The flammability of hydrogen gas can be demonstrated by carefully holding a match or fireplace.
Chemical equation for magnesium oxide and hydrochloric acid. Take Magnesium oxide MgO and Hydrochloric acid HCl. Molecular equation MgOH2s 2HClaq ----- MgCl2aq 2H2Ol Ionic equation- MgOH2s 2Haq 2 View the full answer Transcribed image text.
Chemistry questions and answers. This single replacement reaction is a classic example of a. The reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and release hydrogen gas.
The chemical equation isMgO 2 HCl MgCl2 H2O. The other ions are left unchanged. A Magnesium metal is placed in a solution of hydrochloric acid and reacts to form aqueous magnesium chloride and hydrogen gas 3 marks 2HCl Mg Cle the 6 My 2H 2ch w 2Cl H2 Mg 2 Mg 2t Ma b Aqueous chlorine is.
The balanced chemical equation is. Magnesium can react with aqueous nitric acid HNO3 to form hydrogen gas and magnesium nitrate. What Is Magnesium Hydrochloric Acid Answer Related Questions.
2 Kaq 2 F-aq Mg2aq 2NO 3-aq 2 Kaq 2 NO 3-aq MgF 2 s NIE. H e- rarr 12H_2g And thus the net ionic equation is Mgs 2H rarr Mg2 H_2guarr Overall.
Solved Magnesium Carbonate Magnesium Oxide And Magnesium Hydroxide Are All White Solids That React With Acidic Solutions A Write A Balanced Molecular Equation And A Net Ionic Equation For The Reaction That Occurs

Solved The Net Ionic Equation For The Reaction Between Chegg Com

Solved P 20 Redox Reactions 1 When Magnesium Reacts With Chegg Com
Net Ionic Equation Between Mg Oh 2 And Hcl Mega Lecture Youtube

Solved 2 For Each Of The Following Write I A Balanced Chegg Com

Net Ionic Equation For Mg Hcl Magnesium Hydrochloric Acid Youtube
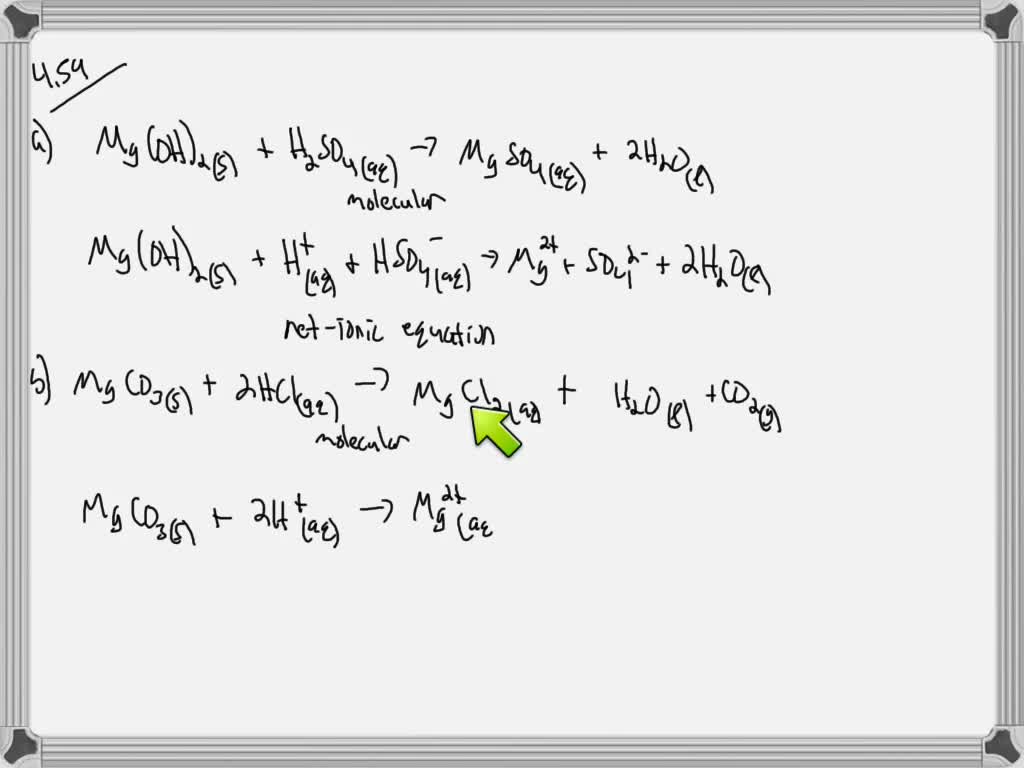
Solved Write Balanced Molecular And Net Ionic Equations For The Reactions Of A Hydrochloric Acid With Nickel B Dilute Sulfuric Acid With Iron C Hydrobromic Acid With Magnesium D Acetic Acid Ch3cooh With

Colour Changes For Simple Indicators Indicatorcolour In Acidcolour In Alkali Litmusredblue Methyl Orangeredyellow Phenolphthaleincolourlessred Ppt Download
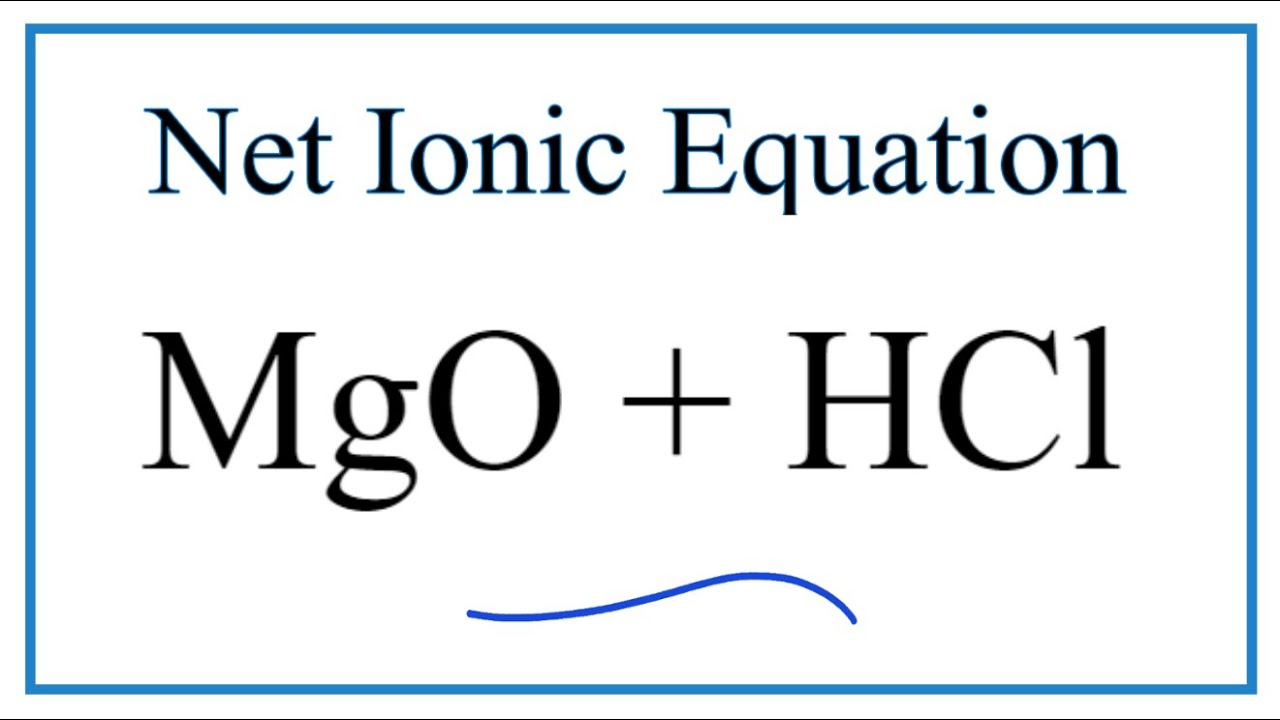
How To Write The Net Ionic Equation For Mgo Hcl Mgcl2 H2o See Note Below Youtube

How To Balance Mg Oh 2 Hcl Mgcl2 H2o Magnesium Hydroxide Hydrochloric Acid Youtube

Water And Hydrogen Comprehensive Tutorial Notes Ppt Download

Writing Ionic Equation Video Lessons Examples And Solutions

Gcse Reactivity Series Of Metals Metallic Activity Order Word Symbol Equations Of Reactions Of Metals With Air Oxygen Water Hydrochloric Acid Sulphuric Sulfuric Acid Nitric Acid Igcse Ks4 Science Chemistry Revision Notes Revising

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download

Question Video Writing A Net Ionic Equation For The Reaction Of Solid Calcium Carbonate With A Hydrochloric Acid Solution Nagwa
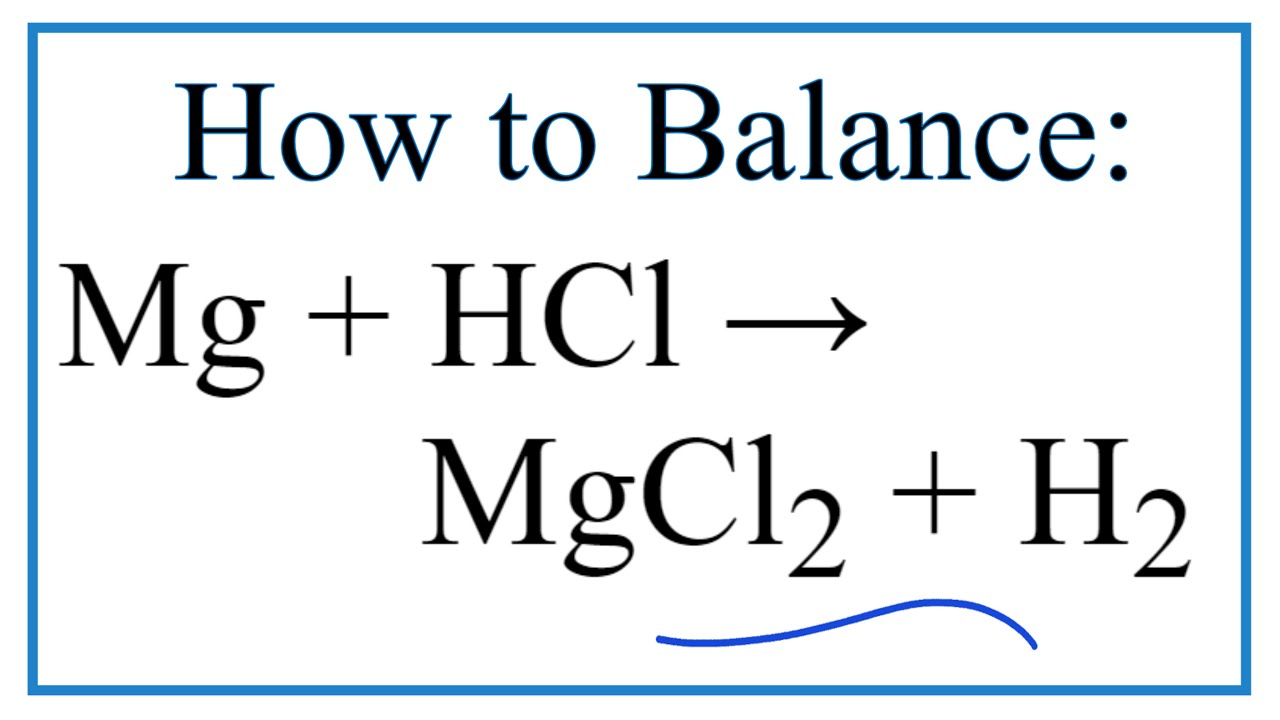
Net Ionic Equation For Mg Hcl Magnesium Hydrochloric Acid Youtube

How To Write The Net Ionic Equation For Mg Oh 2 Hcl Mgcl2 H2o Youtube

Solved The Mineral Dolomite Contains Magnesium Carbonate This Reacts With Hydrochloric Acid Mathrm Mgco 3 Mathrm S 2 Mathrm Hcl Mathrm Aq Rightarrow Mathrm Co 2 Mathrm G Mathrm Mgcl 2 Mathrm Aq Mathrm H 2 Mathrm O

Comments
Post a Comment